Service

In-vitro Efficacy Evaluation
Provide scientific support for relevant cosmetic efficacy claims
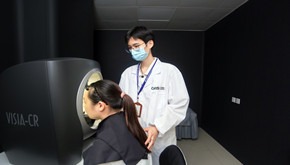
Clinical Efficacy Evaluation
Evaluate the scientificity, authenticity, reliability and traceability of the submitted summary.

Safety Evaluation
Cosmetic registrants and filers should conduct safety assessment by themselves or by entrusting professional institutions
Why choose KEXIN Biotech?

Global Resources
With HQs in China, CIRS Group has branch companies in USA, UK, France, Korea, Japan, Ireland, that can provide cutting edge technical information and support to our clients
Rich Experience
CIRS Group has 16 years accumulation on testing and compliance service technical abilities. CIRS Group has served more than 15,000 clients, including 60+ world top 500 companies
One-Stop-Service Capability
Service range includes cosmetic ingredient development, physical/chemical tests, in-vivo toxicological tests, toxicology risk assessment, in-vitro toxicological study, clinical efficacy study, in-vitro efficacy study, raw material registration, product registration and etc.
Experts Team
Technical team built with experts from personal care industry, College Professor, Risk Assessment Expert, Toxicological Expert, Regulatory Compliance Expert
Professional Equipment and Technique
Capability includes the professional instruments and techniques for in-vitro efficacy study, in-vitro safety study, clinical efficacy study, in-vitro study method development, ingredient development and improvement
Why choose KEXIN Biotech?
01
Global Resources
With HQs in China, CIRS Group has branch companies in USA, UK, France, Korea, Japan, Ireland, that can provide cutting edge technical information and support to our clients
02
Rich Experience
CIRS Group has 16 years accumulation on testing and compliance service technical abilities. CIRS Group has served more than 15,000 clients, including 60+ world top 500 companies
03
Experts Team
Technical team built with experts from personal care industry, College Professor, Risk Assessment Expert, Toxicological Expert, Regulatory Compliance Expert
04
One-Stop-Service Capability
Service range includes cosmetic ingredient development, physical/chemical tests, in-vivo toxicological tests, toxicology risk assessment, in-vitro toxicological study, clinical efficacy study, in-vitro efficacy study, raw material registration, product registration and etc.
05
Professional Equipment and Technique
Capability includes the professional instruments and techniques for in-vitro efficacy study, in-vitro safety study, clinical efficacy study, in-vitro study method development, ingredient development and improvement

